-
Testing & Calibration Laboratories (SAMM)
No Application Kit Download 1 LA 201C/T Application Form DOCX Attachment to LA 201C/T PDF 2 LA 101-5 Application Checklist DOCX 3 LA 201-7 MS ISO/IEC 17025:2017 Checklist DOCX 4 LA 1501-3 PT Record DOCX LA 1501-5 PT Plan DOCX 5 SAMM Leaflet 3 (Recognition) PDF 6 SI-2 Information On Local Proficiency Testing (PT) Provider, Issue 2,1 Feb 2018 PDF 7 Information on SAMM Accreditation PDF No Application Form for Extension of Scope / Extension of Branch Download 1 LA 202EXT (for Testing & Calibration Lab) DOCX 2 LA 202-3 Checklist for Application Extension of Branch DOCX No Application Form for Remote Assessment Download 1 AF 117-1 DOCX -
Medical Testing Laboratories (SAMM MT)
No Application Kit Download 1 Application Kit Cover DOCX 2 Application Form (LA 201 MT) DOCX Info sheet on Application Form DOCX 3 Application Checklist (LA 101- 5 MT) DOCX 4 LA 1501-3 MT ILC Record DOCX LA 1501-5 MT ILC Plan DOCX 5 Public List Of SAMM Documents (LA 101-4 MT) DOCX 6 General Information about accreditation PDF The difference between accreditation and certification PDF The benefits of accreditation PDF No Application Form for Extension of Scope / Satellite Laboratory Download 1 LA 202EXT MT Application form for EOS/Satellite Laboratory DOCX 2 LA 202-3 MT Satellite Application Checklist DOCX No Application Form for Remote Assessment Download 1 AF 117-1 - Remote Assessment Request Form DOCX -
Certification Bodies (ACB)
- Quality Management Systems (QMS)
- Environmental Management Systems (EMS)
- Occupational Safety & Health Management Systems (OSH)
- Information System Management Systems (ISMS)
- Business Continuity Management Systems (BCMS)
- Food Safety Management Systems (FSMS) including FAMI QS Certification
- Good Manufacturing Practices for Food (GMP)
- HACCP Systems (HACCP)
- Food Safety System Certification (FSSC)
- Medical Device Quality Management Systems (MDQMS)
- Energy Management Systems (EnMS)
- Road Transport Safety Management Systems (RTSMS)
- Forest Management Certification (FMC)
- Malaysian Sustainable Palm Oil - Oil Palm Management Certification (MSPO OPMC)
- Anti-bribery Management Systems (ABMS)
- Automotive Certification Scheme (ACS)
-
Product Certification (PC) including Chain of Custody, Ecolabel, Halal and MSPO - Supply Chain Certification (SCC)
No PC including Chain of Custody, Ecolabel, Halal and MSPO Download 1 Application Form View 2 FM 202 Scope of accreditation and sector competence of auditors DOCX 3 FM 205 Checklist for acceptance of application DOCX 4 FM 410 -PC Checklist to ISO/IEC 17065 DOCX FM 410-SR Standards Review Checklist DOCX - Certification of Persons (Persons)
- Adventure Tourism Safety Management Systems (ATSMS)
- Application Form for Extension of Scope
-
Inspection Bodies (MIBAS)
No Application Kit Download 1 Application Form (LA 201 IB) DOCX 2 Application Checklist (LA 101- 5 IB) DOCX 3 LA 1501-3 IB PT Record DOCX 4 LA 1501-5 IB PT Plan DOCX 5 Information on MIBAS Accreditation PDF No Application Form for Extension of Scope Download 1 LA 202 EXT IB DOCX 2 LA 202-3 IB EOB Application Checklist DOCX No Application Form for Remote Assessment Download 1 AF 117-1 DOCX - Test Facilities (GLP CP)
-
Proficiency Testing Provider (MyPTP)
No Application Kit Download 1 PA 101-5 Application Checklist DOCX 2 PA 201 Application Form DOCX 3 Attachment to PA 201 DOCX 4 PA 301- 4 Adequacy Audit Checklist DOCX No Application Form for Extension of Scope Download 1 PA 202 EXT PTP DOCX No Application Form for Remote Assessment Download 1 AF 117-1 DOCX -
Validation & Verification Bodies (AVVB)
No Application Kit Download 1 Application Form View 2 FM 202 Scope of accreditation and sector competence of auditors DOCX 3 FM 205 Checklist for acceptance of application DOCX 4 FM 410 - V&V Checklist to ISO/IEC 17029 DOCX FM 410 - CORSIA Checklist to CORSIA DOCX No Application Form for Extension of Scope Download 1 Extension of Scope Application form View -
Primary Healthcare Laboratories (PHLAS)
No Application Kit Download 1 Application Form DOCX
Accessibility Tools
- Content scaling 100%
- Font size 100%
- Line height 100%
- Letter spacing 100%
Get Accredited
-
SAMM, MIBAS, MyPTP, GLP CP, PHLAS Fees Schedule
Based on Standards of Malaysia (Fees) Regulation 2018, as of 1 January 2019:
Descriptions 1. Application Fees For Each Laboratory/ Inspection Body/ Proficiency Testing Provider/ Test Facilities a) New Application RM 2,000.00 b) Application for Extension of Branch RM 2,000.00 per branch 2. Annual Renewal Fees For Each Laboratory/ Inspection Body/ Proficiency Testing Provider/ Test Facilities For Each Additional Branch RM 1,000.00 RM 500.00 3. Assessment Fees a) Assessment Fees for Assessor Team Leader/Lead Inspector RM 1,000.00 per day or RM 500.00 (if less than 4 hours) Assessor/Technical Assessor/Inspector RM 800.00 per day or RM 400.00 (if less than 4 hours) b) Witnessing assessment (For MIBAS only) RM 800.00 per day or RM 400.00 (if less than 4 hours) 4. Overseas assessor contracted by Standards Malaysia (decision of 10th Malaysian Accreditation Council meeting 10.09.96) Actual professional charges, cost of return flights (business class) and accommodation arising from engaging overseas assessors will be charge directly to the laboratory. 5. Assessors contracted by Standards Malaysia to perform assessments overseas by quotation 6. Appeal Fee RM 1,500.00 Application fee is non-refundable. An application is considered lapsed if the applicant fails to obtain accreditation within two years from the date of acceptance of application.
-
ACB and AVVB Fees Schedule
Based on Standards of Malaysia (Fees) Regulation 2018, as of 1 January 2019:
Descriptions 1. Application Fees a) New Application RM 2,000.00 b) Application for additional programme RM 2,000.00 2. Annual Renewal Fees RM 2,000.00 per programme 3. Assessment Fees a. Assessment Fees for Assessor Team Leader RM 1,500.00 per day or RM 750.00 (if less than 4 hours) Assessor RM 1,200.00 per day or RM 600.00 (if less than 4 hours) Technical Expert RM 1,000 per day or RM 500.00 (if less than 4 hours) b) Witnessing assessment RM 1,000.00 per day or RM 500.00 (if less than 4 hours) 4. Overseas assessor contracted by Standards Malaysia (decision of 10th Malaysian Accreditation Council meeting 10.09.96) Actual professional charges, cost of return flights (business class) and accommodation arising from engaging overseas assessors will be charge directly to the ACB / AVVB. 5. Assessors contracted by Standards Malaysia to perform assessments overseas by quotation 6. Appeal Fee RM 1,500.00 Application fee is non-refundable. An application is considered lapsed if the applicant fails to obtain accreditation within two years from the date of acceptance of application.
-
Testing & Calibration Laboratories (SAMM)
 Laboratory accreditation based on ISO/IEC 17025 (formerly ISO/IEC Guide 25) was first introduced in Malaysia on 1 July 1987. This was followed by the introduction on 15 August 1990 of a national unified laboratory accreditation scheme, known as Skim Akreditasi Makmal Malaysia (SAMM).
Laboratory accreditation based on ISO/IEC 17025 (formerly ISO/IEC Guide 25) was first introduced in Malaysia on 1 July 1987. This was followed by the introduction on 15 August 1990 of a national unified laboratory accreditation scheme, known as Skim Akreditasi Makmal Malaysia (SAMM).Following the establishment of the Department of Standards on 28 August 1996 under the Standards of Malaysia Act, 1996, all accreditation activities of the former Malaysia Accreditation Council were transferred to and come directly under the responsibility of Department of Standards Malaysia.
Laboratory accreditation based on ISO 15189 for medical testing laboratories was first introduced in Malaysia on 14 December 2004.
-
-
FIELDS OF TESTING
No Title Details 1 Chemical Chemical tests and analysis on products and materials. 2 Biological Biological, microbiological and biomedical, testing and measurement, including examinations of foods, drugs and pharmaceuticals. 3 Electrical Electrical testing covers tests of an essentially electrical nature including radio frequency (RF) and electromagnetic compatibility (EMC) performed on materials, component, devices, appliances and equipment. 4 Thermal Including thermal characteristics of building materials, fire testing such as tests evaluating fire resistance, ignitability, flammability, etc., of products and materials. 5 Mechanical Mechanical and physical testing of material / products that include metallurgical tests to determine the elemental analysis and microstructures. 6 Non-Destructive Testing (NDT) Examination of material, component and assembly to detect discontinuities without damaging the material, component or assembly. Tests include radiography, ultrasonic, penetrants, magnetic particle and eddy currents. 7 Radioactivity Testing Examination of material, component and assembly to detect discontinuities without damaging the material, component or assembly. Tests include radiography, ultrasonic, penetrants, magnetic particle and eddy currents. 8 Household Pesticide Includes testing on the following scopes; mosquito mats and electric liquid vaporizer, space spray aerosol, residual spray aerosol, direct spray aerosol, mosquito coils, cockroach baits, mosquito skin repellent, household rat baits, smokeless paper mosquito coils, mosquito gels an other similar products. 9 Toxicity Testing for chemical products, manufactured products, cosmetic and skin care products, medical devices and also wastes and environmental samples. 10 Electromagnetic Compatibility (EMC) Testing for electromagnetic compatibility (EMC) including electromagnetic disturbance test and immunity test. 11 Veterinary Includes testing on the following scopes; pathology, clinical pathology, bacteriology & mycology, virology, parasitology, toxicology, feed analysis, water quality analysis, biological. 12 Genetically Modified Organism (GMO) Analysis for detection and quantification of GMO covers both DNA and protein based methods. 13 Nucleic Acid Requirements for accreditation of laboratories involved in nucleic acid testing in a broad variety of sample that provide services in particular fields related to molecular biology and/or genetic analysis. 14 DNA Profiling For Forensic Science Comprises of DNA Profiling for forensic DNA profiling and paternity testing using DNA method. 15 Analysis Of Accelerant In Fire Debris For Forensic Science Includes testing for fire accelerants in fire debris for forensic science testing laboratories. 16 Document Examination for Forensic Science Requirements for accreditation of questioned document examination for forensic science testing laboratories. 17 Information Technology Security Evaluation and Testing: Common Criteria Requirements on functional and assurance of ICT products and systems, which provide a common baseline for security evaluation. 18 Software Testing Requirement for accreditation of software testing. -
FIELDS OF CALIBRATION
No Title Details 1 Heats And Temperature Measurements Including heat, temperature and humidity measuring equipment. 2 Electrical Measurements Including the calibration of electrical and electronic instruments and equipment. 3 Mass And Mass-Related Quantities Measurements Including measurement of mass, density, pressure, force, hardness, viscosity, flow, and volume and the examination of machines and instruments used in these measurements. 4 Optical And Photometric Measurements Including measurement made with and on optical and photometric equipment and instruments: measurement of colour and surface smoothness (reflectance, gloss); measurements involving visible (light) and near-visible (infrared, ultra violet) wavelength of radiation. 5 Dimensional Measurement Including various length and dimensional calibrations work. 6 Acoustic & Vibration Measurement Including measurement of environmental noise and mechanical vibration, calibration of acoustic and vibration measuring equipment, acoustic and vibration characteristics of materials and structures, audiometry, measurement of sound power, acoustic and vibration performance tests and dynamic balancing 7 Radioactivity Measurement Including the calibration of radiation measuring equipment. -
FIELDS OF MEDICAL TESTING LABORATORIES (SAMM MT)
No Title Details 1 Anatomical Pathology (Cytopathology) - Gynaecological Cytopathology (GYN Cytopathology)
i) Conventional
ii) Liquid based - Non-Gynaecological Cytopathology (Non-GYN Cytopathology)
- Fine Needle Aspiration Cytology (FNAC)
- Specialised tests such as special stains, immunohistochemical stains and molecular testing (HPV DNA) if performed in the cytopathology laboratory shall be included in the scope of accreditation.
2 Anatomical Pathology (Histopathology) - Diagnostic Histopathology
- Intraoperative Frozen Sections
All ancillary tests (e.g. immunohistochemistry) performed in the laboratory and incorporated in the histopathology report shall be part of the accreditation scope.
3 Chemical Pathology - General chemistry for blood, urine and body fluids
- Tumor markers
- Hormones
- Quantitative immunology testing
- Therapeutic drug monitoring
- Special proteins/specific proteins
- Biogenic amines
- Clinical toxicology, heavy metals and trace elements
- Drugs of abuse testing
- Biochemical genetics testing
4 Haematology - Routine haematology may include Full blood count and differential count, Peripheral blood film examination, Reticulocyte count, Erythrocyte sedimentation rate, Prothrombin time / International Normalised Ratio, Activated partial thromboplastin time, Thrombin time, D-dimer / Fibrinogen degradation products, Fibrinogen and Glucose-6-phosphate dehydrogenase deficiency (G6PD) screening.
- Specialised haematology may include Full blood picture with clinical interpretation, Haemoglobin analysis, Bone marrow examination, Special haemostasis e.g. factor assays, lupus anticoagulant testing,
thrombophilia screening, Flow cytometric examination e.g. CD4/CD8 enumeration, CD34 enumeration, leukemia/lymphoma immunophenotyping, paroxysmal nocturnal haemoglobinuria (PNH) testing, and Molecular testing- Blood transfusion tests may include Blood grouping and phenotyping, Antibody screening and identification, Compatibility testing, Investigation of transfusion reaction, and Direct Antiglobulin Test (DAT)
5 Medical Microbiology - Bacteriology
- Virology
- Parasitology
- Mycology
- Mycobacteriology
- Immunology
6 Assisted Reproductive Technology (ART) - Semen Analysis (recognized standards e.g. WHO)
- Sperm preparation (Fresh sample/frozen sample/MESA/PESA/TESA/Open Biopsy)
- Sperm cryopreservation
- In Vitro Fertilization (IVF)
- Gamete Intra Fallopian Transfer (GIFT)
- Intra Cytoplasmic Sperm Injection (ICSI)
- Assisted Hatching
- Oocyte/Embryo/blastocyst cryopreservation
7 Cytogenetics - Constitutional Cytogenetics (Prenatal & Postnatal)
- Cancer Cytogenetics
- Fluorescence In Situ Hybridisation (FISH)
8 Medical Molecular Testing - Inherited diseases
- Cancer genetics
- Infectious diseases
- Immunogenetics
- Pharmacogenomics
- Gynaecological Cytopathology (GYN Cytopathology)
-
FIELDS OF TESTING
-
-
Certification Bodies (ACB)
Department of Standards Malaysia, under the purview of The ACB Scheme (Scheme for the Accreditation of Certification Bodies ACB), is responsible for the assessment and accreditation of certification bodies. Certification bodies are bodies that provide conformity certificates to organisations for their various management systems, individual as well as product manufacturers and service providers.
-
Presently, programmes offered under the ACB Scheme are as follows:
- Quality Management Systems (QMS) against criteria
ISO/IEC 17021-1, ISO/IEC 17021-3 & related IAF MD Series
- Environmental Management Systems (EMS) against criteria
ISO/IEC 17021-1, ISO/IEC 17021-2 & related IAF MD Series
- Occupational Safety and Health Management Systems (OSH) against criteria
ISO/IEC 17021-1, ISO/IEC TS 17021-10, IAF MD 22 & other related IAF MD Series
- Information Security Management Systems (ISMS) against criteria
ISO/IEC 17021-1, ISO/IEC 27006 & other related IAF MD Series
- Information Security Management Systems (ISMS) for Cloud Service Providers against criteria
ISO/IEC 17021-1, ISO/IEC 27006, ISO/IEC 27017, ISO/IEC 27018 & other related IAF MD Series
- Medical Device Quality Management System (MDQMS) against criteria
ISO/IEC 17021-1, IAF MD 9 & other related IAF MD Series
- Energy Management System (EnMS) against criteria
ISO/IEC 17021-1, ISO 50003, ACB-EnMS & related IAF MD Series
- Forest Management Certification (FMC) against criteria
ISO/IEC 17021-1, ACB-FMC - Series for Natural Forests & Forest Plantations & related IAF MD Series
- Good Manufacturing Practice for Food (GMP) against criteria
ISO/IEC 17021-1, ACB-GMP & related IAF MD Series
- HACCP Systems against criteria
ISO/IEC 17021-1, ACB-HACCP & related IAF MD Series
- Food Safety Management Systems (FSMS) against criteria
ISO/ IEC 17021-1, ISO/TS 22003 & other related IAF MD Series
- Animal Feed Certification Programme based on FAMI QS against criteria
ISO/ IEC 17021-1, ISO/TS 22003 & FAMI QS Rules for Certification Bodies & other related IAF MD Series
- Food Safety System Certification (FSSC 22000) against criteria
ISO/IEC 17021-1, ISO/TS 22003, FSSC 22000 Requirements & other related IAF MD Series
- Product Certification (PC) Systems against criteria
ISO/IEC 17065 & related IAF MD Series
- PC - Chain of Custody (CoC) against criteria
ISO/IEC 17065, PEFC ST 2003 & related IAF MD Series
- PC - Ecolabel against criteria
ISO/IEC 17065 & ISO 14024 (Type I) & ACB-Ecolabel & related IAF MD Series
- Automotive Certification Scheme
ISO/IEC 17021-1, Scheme Owner Requirements & related IAF MD Series
- Certification of Persons (PERSONS) against criteria
ISO/IEC 17024 & related IAF MD Series
- Malaysian Sustainable Palm Oil (MSPO) - Oil Palm Management Systems
ISO/IEC 17021-1, ACB OPMC series, scheme owner requirements & IAF MD Series
- Malaysian Sustainable Palm Oil (MSPO) - Supply Chain Certification (SCC)
ISO/IEC 17065, scheme owner requirements & related IAF MD Series
- Anti-Bribery Management Systems (ABMS)
ISO/IEC 17021-1, ISO/IEC 17021-9, scheme owner requirements & related IAF MD Series
- Road Transport Safety Management Systems (RTSMS)
ISO/IEC 17021-1, ISO/IEC TS 17021-7 & related IAF MD Series
- Business Continuity Management Systems (BCMS)
ISO/IEC 17021-1, ISO/IEC TS 17021-6 & related IAF MD Series
- Validation & Verification Scheme (V&V)
ISO/IEC 17029, ISO 14065 & related IAF MD Series
- Validation & Verification Scheme (V&V) - Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA)
ISO 14065, ICAO SARPS Annex 16 Volume 4, ICAO ETM & related IAF MD Series
- Adventure Tourism Safety Management Systems
ISO/IEC 17021-1, ISO/IEC 17021-10 & related IAF MD Series
- Quality Management Systems (QMS) against criteria
-
-
Inspection Bodies (MIBAS)
MIBAS pronounced as [my bess] - Malaysia Inspection Bodies Accreditation Scheme was launched on 20 November 2006 which was based on ISO/IEC 17020.
Inspection is the examination of a product design, product service, process or plant and determination of conformity with specific requirements or based on professional judgement. This broad definition indicates the variety of inspection activities that exist.
-
No Title Details 1 Agriculture and Agricultural Meats products, Milk and dairy products, Grain products / Pepper, Palm oil and Rubber, Fish and Fishery products. 2 Industrial and Commercial Construction and Maintenance Chemical and petrochemical processing plant, Power generation plant, Power transmission plant, Pipelines, Coatings and corrosion, Welding Product identification and traceability. 3 Bulding Construction and Maintainance Commercial buildings, Steel fabricated structures & assemblies, Fire protection equipment, Corrosion & Coatings, Safety measurement equipment. 4 Industral Equipment and Machinery Pressure equipment, Cranes, elevators, conveyors and lifting gear, Agriculture, mining and earth moving equipment, Industrial machinery, Electric generators, motors and related equipment, Coatings and corrosion, Welding, Construction equipment, Fabricated assemblies and structures, Product identification and traceability, NDT. 5 Proces Inspection Welding, Coatings, Packaging, Labeling, Product identification, Product traceability, Process readiness, Expediting, Witnessing, process surveillance, Preshipment. 6 Manufactured Goods Fabricated metal components and products, Electrical and electronic products, Food and beverages,Refractories and ceramics, Product identification and traceability. 7 Natural Resources and Refined Products Fabricated metal components and products, Electrical and electronic products, Food and beverages, Refractories and ceramics, Product identification and traceability.
-
-
Test Facilities (GLP CP)
Standards Malaysia is one of the CMAs appointed by Cabinet of Malaysia on 13 February 2008 as the Compliance Monitoring Authority (CMA) for monitoring compliance with the Organisation for Economic Co-operation and Development Principles of Good Laboratory Practice (OECD GLP).
As GLP CMA, Standards Malaysia has adopted the OECD Principles of GLP. The structure, policies and procedures under which Standards Malaysia operates are documented to ensure implementation of these policies and procedures are administered in an independent and impartial manner to ensure the smooth operation of all compliance activities. Standards Malaysia quality system has been established, documented, implemented and maintained to give confidence in its ability to operate the compliance process in an effective manner.
-
GLP CP is a voluntary programme open to test facilities conducting studies for non-clinical health and environmental safety studies and for purpose of registering and/or licensing on test item contains in product in the following categories:
- industrial chemicals;
- feed additives;
- pesticides;
- biotechnology (non-pharmaceuticals);
- others
These test items are frequently synthetic chemicals, but may be of natural or biological origin and, in some circumstances may be living organisms. The purpose of the non-clinical safety testing of test items is to obtain data on their properties and/or their safety with respect to human health and the environment. Non-clinical health and environment safety studies covered by the Principles of Good Laboratory Practice include work conducted in the laboratory, in greenhouses, and in the field.
Type of studies/area of expertise on test item subjected to Standards Malaysia GLP CP in the following broad categories:
- physical-chemical testing
- toxicity studies
- mutagenicity studies
- environmental toxicity studies on aquatic and terrestrial organisms
- studies on behavior in water, soil and air; bioaccumulation
- residue studies
- studies on effects on mesocosms and natural ecosystems
- analytical and clinical chemistry testing
- others
Note: Test facility conducting studies on pharmaceutical, cosmetics, food additives and veterinary drugs will be inspected by National Pharmaceutical Regulatory Agency (NPRA), Ministry of Health.
-
-
Proficiency Testing Provider (MyPTP)
Malaysia Proficiency Testing Provider Accreditation Scheme (MyPTP) which based on ISO/IEC 17043 ‘Conformity assessment - General requirements for proficiency testing' was launched on 31 December 2013.
The scheme provides accreditation service to ensure the Proficiency Testing Provider's competency that provides proficiency testing programmes in area of testing, calibration, medical testing and inspection. Proficiency testing usually involves the use of inter-laboratory comparisons for determination of participants' (laboratories and inspection bodies) performance.-
-
Fields of Testing
- Chemical
- Biological
- Electrical
- Thermal
- Mechanical
- Non-Destructive Testing (NDT)
- Radioactivity
- Veterinary
- Forensic Science
- Information and Communication Technologies (ICT)
-
Fields of Calibration
- Heat and Temperature
- Electrical
- Mass and Mass-Related Quantities (density, pressure, force, torque, hardness, viscosity, flow, and volume)
- Optical and Photometric
- Dimensional
- Acoustic & Vibration
- Radioactivity
-
Fields of Medical Testing
- Anatomical Pathology (Cytopathology)
- Anatomical Pathology (Histopathology)
- Chemical Pathology
- Haematology
- Medical Microbiology
- Virology
- Assisted Reproductive Technology
- Cytogenetics
-
Fields of Inspection
- NDT Inspection
- Welding Inspection
- Vehicle Inspection
- Shop Inspection
- Manufactured Goods
- Factory Audits
- Electrical Products
-
Fields of Testing
-
-
Validation & Verification Bodies (AVVB)
Department of Standards Malaysia (JSM), under the purview of The ACB Scheme (Scheme for the Accreditation of Certification Bodies ACB), is responsible for the assessment and accreditation of validation and/or verification bodies. Validation and/or verification bodies are bodies that providing confirmation that claims are either plausible with regards to the intended future use (validation) or truthfully stated (verification).
-
Presently, programmes offered under the AVVB Scheme are as follows:
- ISO 14064-1 (Includes GHG Protocol)
- Power generation and electric power transactions
- General manufacturing (physical or chemical transformation of materials or substances into new products)
- Oil and gas exploration, extraction, production and refining, and pipeline distribution, including petrochemicals
- Metals production
- Aluminum production
- Mining and mineral production
- Pulp, paper and print
- Chemical production
- Carbon capture storage
- Transport
- Waste handling and disposal
- Agriculture, Forestry and Other Land Use (AFOLU)
- General
- ISO 14064-2
- Combustion of fossil fuels
- Process emissions (chemical reactions linked to the process industries such as cement, pulp and paper, glass, steel)
- Agriculture, forestry and other land use
- Livestock
- Carbon capture and storage
- Waste management
- CORSIA
-
-
Primary Healthcare Laboratories (PHLAS)
The MS 2702: Primary healthcare laboratories - Requirements for quality and competence was approved by Minister of International Trade and Industries (MITI) on 8th October 2020. The PHLAS has been launched on 1st February 2021.
-
The accreditation scheme is voluntary scheme open to any primary healthcare laboratories that provides conformity assessment services for medical testing such as Haematology, Chemical Pathology, Medical Microbiology and others.
-
-
Proposal For New Accreditation Proposal (Scheme / Programme / Field)
Can't find what are you looking for? Do you want to establish a new accreditation field?
Please download the attachment and send to This email address is being protected from spambots. You need JavaScript enabled to view it.:
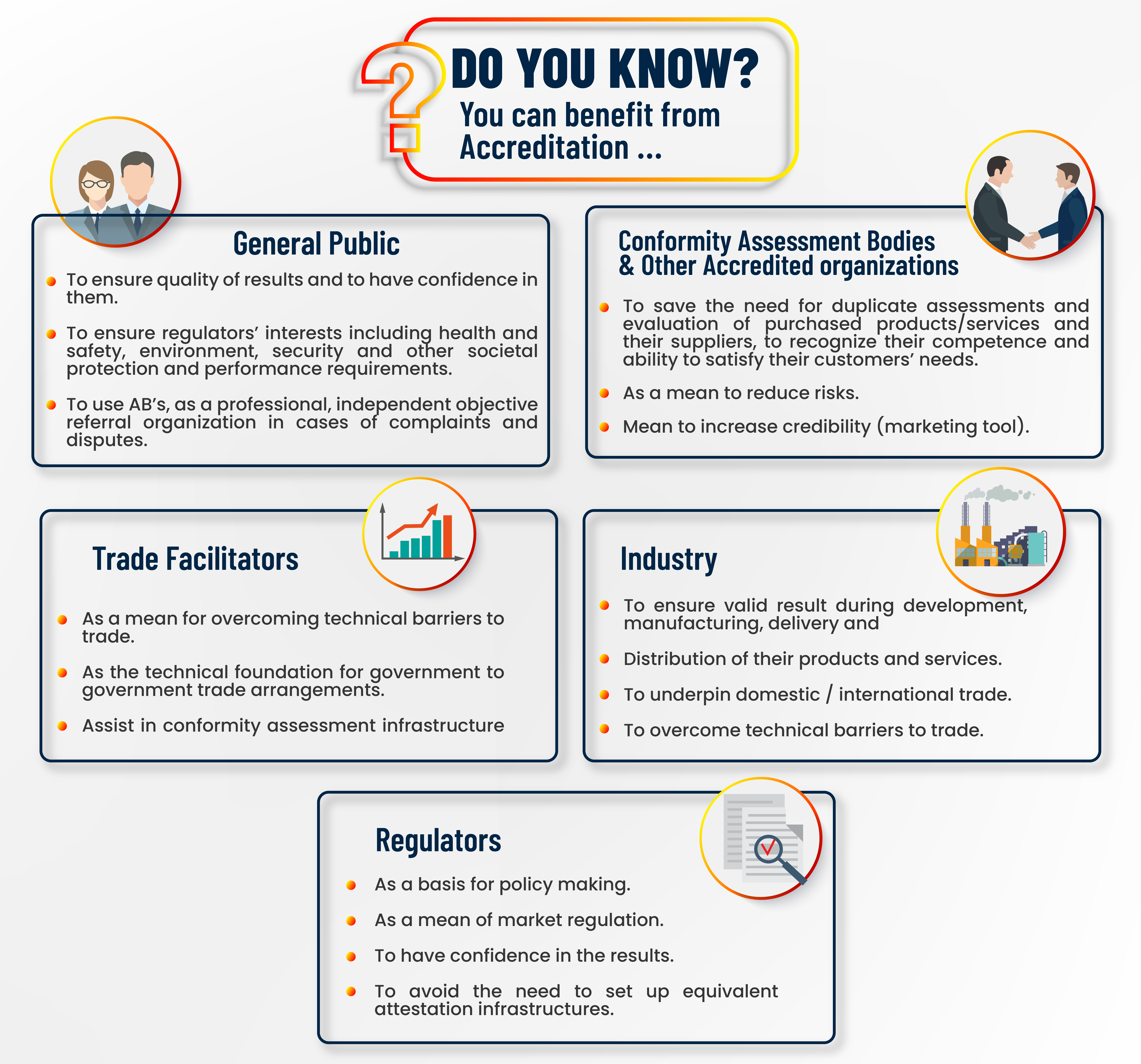
Accreditation enhances confidence in certificates and conformity statements. Accreditation enchances quality of results by ensuring their traceability, comparability validity and commutability.
Accreditation is essential for ensuring credibility, integrity and competency of conformity assessment bodies as well as others in facilitating their functions and services.
CONTACT US
Level 4-7, Tower 2Menara Cyber Axis
Jalan Impact, Cyber 6
63000 Cyberjaya
Selangor, MALAYSIA
Tel : +603-8008 2900
Fax : +603-8008 2901
Email : central[at]jsm[dot]gov[dot]my
VISITOR COUNTER
Today 296
Yesterday 1162
This Week 11824
This Month 296
Total 923799